Carbon atom in alkanes has sp3 hybridization and thus has the preferred angles with its neighbor atoms of about 109 degrees. However, in cyclo-alkanes particularly the small ones such as cyclo-propane the CCC angles are much smaller than its preferred value. As shown below the localized MO of CC bond in cyclo-propane does not have the normal overlap of two sp3 hybrid orbitals.
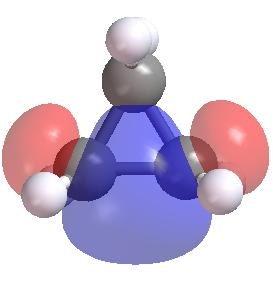
This leads to a significant ring strain energy (RSE) in small cyclo-alkanes and consequently affects the stability and reactivity of these molecules.
In this experiment, you will use two different theoretical approaches, namely isodesmic and homodesmotic reaction approaches to quantify the ring strain energies for a number of cyclo-alkanes.
Isodesmic Reaction Approach
Isodesmic reaction conserves the number of bonds in a given type. For example, for cyclobutane, the isodesmic reaction that can represent the ring strain energy is defined as
☐ + 4 CH4 → 4 H3C━CH3
and thus
RSE = 4 Hf(C2H6) - {Hf(☐) + 4 Hf(CH4)}
where Hf is the heat of formation. Note that the above reaction is balanced in the bond type but not in the bond pattern at each C atom, i.e. local bonding environment with its neighbors. For the above reaction, the reactant side has 4 carbon atoms each has two C-C and two C-H bonds and 4 carbon atoms each has 4 C-H bonds. On the product side, it has 8 carbon atoms each as 3 C-H and 1 C-C bonds. Thus, the nature of the bonding to its neighbor is not preserved.
Homodesmotic Reaction Approach
Homodesmotic reaction preserves the bonding pattern of each carbon and heavy atom. In this case, the homodesmotic reaction representing the ring strain energy of cyclo-butane is:
☐ + 4 H3C━CH3 → 4 H3C-CH2-CH3
RSE = 4 Hf(C3H8) - {Hf(☐) + 4 Hf(C2H6)}
Procedure: Using tools in Avisto. You can download Avisto and its tools from Astonis.
- Use MolLib to send out 3D structures of cyclo-propane to cyclo-octane and then use MolDesign to create CH4 and H3-CH3.
- Use Basic QChem Edu, Basic QChem, Mopac GUI Cloud, or Mopac GUI Pro to search for stable structures of these molecules. Record heat of formation for each species and the CCC angles of cyclo-alkanes.
- Use the formula above to calculate RSE using the isodesmic and homodesmotic reaction approaches.
- Plot the RSE calculated from both approaches versus the averaged CCC angle and deviation from the ideal 109.7 degreesfor each species.
- Compare your values to those derived from experimental heats of formation below (kcal/mol).
| Species | Isodesmic | Homodesmotic |
| cyclo-propane | 19.48 | 27.72 |
| cyclo-butane | 15.77 | 26.77 |
| cyclo-pentane | -7.03 | 6.72 |
| cyclo-hexane | -16.01 | 0.48 |
| cyclo-heptane | -12.50 | 6.74 |
| cyclo-octane | -11.76 | 10.23 |
- Draw your conclusion on RSE and accuracy of the isodesmic and homodesmotic reaction approaches.



